A team of scientists staffed with researchers from Brookhaven National Laboratory and SLAC National Accelerator Laboratory have discovered why cathodes in lithium-ion batteries degrade, as well as possible remedies. This could lead to more affordable and better-performing batteries for electric vehicles.
For electric vehicles to deliver the same reliability as gas vehicles, they need lightweight-yet-powerful batteries. Lithium-ion batteries are the most common type of battery used in electric vehicles, but their high cost and limited lifetimes limit widespread deployment of electric vehicles. To overcome these challenges, scientists at many of DOE’s national labs are researching ways to improve traditional lithium-ion batteries.
“Layered materials are relatively easy to synthesize and have high-capacities and energy densities,” says Brookhaven chemist Enyuan Hu.
Lithium cobalt oxide, for example, is a layered material that has been used as the cathode for lithium-ion batteries for many years. Despite its successful application in small energy storage devices such as portable electronics, cobalt’s cost and toxicity limit its use in larger devices. So researchers are investigating how to replace cobalt with safer and more affordable elements without compromising the material’s performance.
“We chose a nickel-rich layered material because nickel is less expensive and toxic than cobalt,” Hu says. “However, the nickel-rich layered materials start to degrade after several charge-discharge cycles in a battery. We want to pinpoint the cause of this degradation and provide possible solutions.”
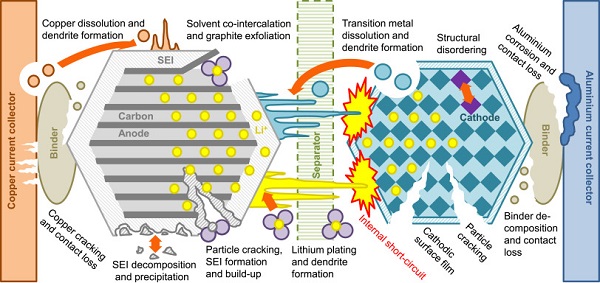
Cathode materials degrade in several ways. For nickel-rich materials, the problem is mainly capacity fading—a reduction in the battery’s charge-discharge capacity after use. To fully understand this process in their nickel-rich layered materials, the teams needed to use several research techniques to assess the material from different angles.
“This is a complex material. Its properties can change at different rates during cycling,” Hu says. “We needed to understand how the material’s structure changed during the charge-discharge process both physically on the atomic scale and chemically, which involves several elements including nickel, cobalt, manganese, oxygen, and lithium.”
To do so, Hu and his colleagues characterized the material using resources from a handful of research facilities, including the two synchrotron light sources at the National Synchrotron Light Source II at Brookhaven and the Stanford Synchrotron Radiation Lightsource at SLAC.
Results from the x-ray absorption spectroscopy experiments at Brookhaven led researchers to conclude the material had a strong structure that did not release oxygen from the bulk, challenging previous beliefs. Instead, the researchers identified that the strain and local disorder in the cathode material was mostly associated with nickel.
To investigate further, the team conducted transmission x-ray microscopy experiments at SLAC, mapping out the chemical distributions in the material. This created a large set of data, so the scientists applied machine learning to sort through the data.
“The major conclusion we drew from this experiment was that there were considerable inhomogeneities in the oxidation states of the nickel atoms throughout the particle,” says Hu. “Some nickel within the particle maintained an oxidized state, and likely deactivated, while the nickel on the surface was irreversibly reduced, decreasing its efficiency.”
Additional experiments revealed small cracks formed within the material’s structure.
“During a battery’s charge-discharge process, the cathode material expands and shrinks, creating stress,” Hu explains. “If that stress can be released quickly then it does not cause a problem but, if it cannot be efficiently released, cracks can form.”
The scientists theorize that they could possibly mitigate this problem by synthesizing a new material with a hollow structure. They tested and confirmed that theory experimentally, as well as through calculations. Moving forward, the team plans to continue developing and characterizing new materials to enhance efficiency.
“We work in a development cycle,” Stavitski says. “You develop the material, then you characterize it to gain insight on its performance. Then you go back to a synthetic chemist to develop an advanced material structure, and then you characterize that again. It’s a pathway to continuous improvement.”
Additionally, as the DoE labs continues to build up capabilities, scientists plan to complete more advanced experiments on these materials.